Hydroxyl Group – A Journey Into Chemical Marvels!
5 min read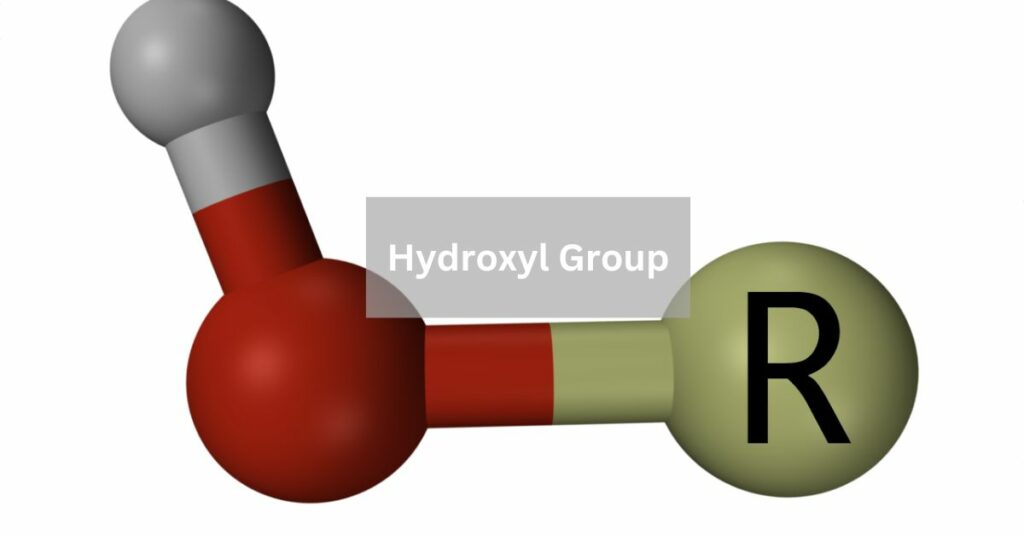
In the vast landscape of organic chemistry, few functional groups are as ubiquitous and versatile as the hydroxyl group. In biology, industry, and the environment, this unassuming chemical moiety plays a fundamental role in oxygen-hydrogen bonding.
The hydroxyl group, denoted as -OH, is a functional group consisting of an oxygen and a hydrogen atom bonded to a carbon atom. It imparts properties like polarity and reactivity to organic molecules.
In this exploration, we delve deep into the significance, properties, and applications of the hydroxyl group, uncovering its remarkable contributions to science and society.
Understanding The Hydroxyl Group – Unravel Its Secrets!
The hydroxyl group, comprising an oxygen atom bonded to a hydrogen atom through a covalent bond, inherently influences the properties of compounds it is a part of.
A key attribute stems from its ability to engage in hydrogen bonding, where the hydrogen atom within the hydroxyl group forms attractive interactions with electronegative atoms like oxygen or nitrogen.
This propensity for hydrogen bonding imparts distinctive traits to hydroxyl-containing compounds, including higher boiling points, enhanced solubility in water, and increased surface tension.
This unique arrangement of the hydroxyl group fosters distinctive properties in the compounds it is found in.
A significant aspect is its capacity for hydrogen bonding, where the hydrogen atom of the hydroxyl group forms attractive interactions with other electronegative atoms such as oxygen or nitrogen.
This tendency toward hydrogen bonding confers special characteristics upon hydroxyl-containing compounds, such as higher boiling points, augmented solubility in water, and elevated surface tension.
Properties And Characteristics – Explore The Essence Of Hydroxyl!
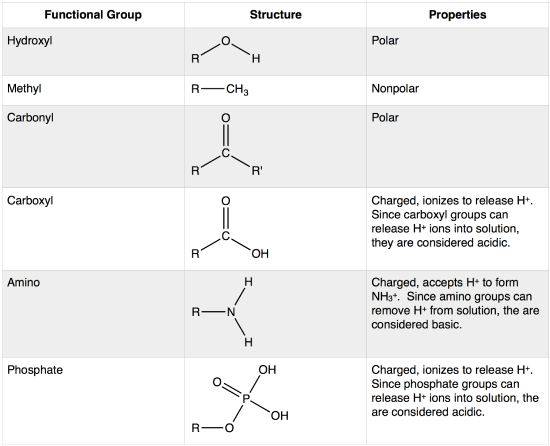
The properties of compounds containing hydroxyl groups vary depending on their chemical context and structural arrangement.
For instance, alcohols, which are organic compounds characterized by the presence of one or more hydroxyl groups, exhibit a wide range of physical and chemical properties.
Small alcohols such as methanol and ethanol are highly volatile liquids with characteristic odors and are commonly used as solvents, disinfectants, and fuels.
In contrast, larger alcohols such as octanol and dodecanol are viscous liquids or waxy solids with limited solubility in water, finding applications in the production of surfactants, emulsifiers, and cosmetics.
Biological Significance – Discover The Wonders Of Hydroxyl Group!
In the realm of biology, the hydroxyl group holds significant importance as a key component of vital biomolecules.
Carbohydrates, for example, which are fundamental sources of energy and structural elements in living organisms, frequently feature multiple hydroxyl groups.
These hydroxyl groups actively participate in intermolecular interactions, such as hydrogen bonding, thus exerting a notable influence on the structure, solubility, and overall function of these biomolecules.
Additionally, hydroxyl groups are essential constituents of nucleic acids (DNA and RNA), proteins, and lipids, each playing crucial roles in various biological processes.
Within nucleic acids, hydroxyl groups contribute to genetic information storage and transfer. In proteins, they aid in proper folding, which is essential for their functional activity.
Moreover, hydroxyl groups found in lipids contribute to cellular signaling processes, modulating various cellular responses and functions.
Overall, the hydroxyl group’s presence in essential biomolecules underscores its significance in biological systems, influencing diverse aspects ranging from molecular structure to cellular function.
Its versatile roles highlight the intricate interplay between chemical functionality and biological processes, underscoring the importance of understanding molecular interactions in biological contexts.
Integrate Hydroxyl For Industrial Success – Achieve Excellence!
The versatility of the hydroxyl group extends to numerous industrial applications, ranging from the production of polymers to the synthesis of pharmaceuticals.
Polyols, which are compounds with multiple hydroxyl groups, serve as building blocks for the synthesis of polyurethanes, polyester resins, and other polymers.
Additionally, the hydroxyl group serves as a functional moiety in the synthesis of pharmaceutical compounds such as antibiotics, analgesics, and antiviral drugs.
By leveraging the unique reactivity of hydroxyl groups, chemists can design and fabricate a diverse array of materials and compounds with tailored properties and functionalities.
Environmental Implications Of Hydroxyl Group – Join The Green Revolution!
The presence of hydroxyl groups in environmental contaminants and pollutants highlights their pivotal role in environmental chemistry and remediation efforts.
Hydroxylated polychlorinated biphenyls (OH-PCBs), for example, are harmful byproducts of industrial activities, presenting significant threats to both human health and the environment.
It is imperative to grasp their chemical attributes, reactivity, and interactions with environmental components to comprehend the behavior and movement of OH-PCBs within the environment.
Furthermore, hydroxyl radicals (·OH), characterized by their high reactivity, are generated through processes like the photolysis of water and hold a crucial position in atmospheric chemistry.
They actively participate in the breakdown of pollutants, contributing substantially to their degradation, while also participating in the creation of secondary pollutants such as ozone.
Understanding the mechanisms involving hydroxyl radicals is paramount in managing and mitigating the impact of pollutants on the environment and human health.
Future Perspectives – Embrace the future with us!
As scientific understanding and technological capabilities advance, the hydroxyl group continues to captivate researchers across diverse fields, spanning materials science to biotechnology.
By delving into the nuanced mechanisms governing hydroxyl-containing compounds, scientists strive to unlock novel materials, therapies, and technologies with profound implications for human health, sustainability, and economic prosperity.
The breadth of potential applications, from renewable energy sources to biodegradable polymers, underscores the limitless possibilities offered by harnessing the versatility of the hydroxyl group.
Elucidating the intricate behavior of hydroxyl-containing compounds fuels a surge of exploration and innovation in the scientific realm.
Researchers aim to decipher the underlying mechanisms, paving the way for groundbreaking discoveries that transcend disciplinary boundaries.
With each revelation, the horizon of possibilities expands, offering tantalizing glimpses into a future where hydroxyl-derived advancements revolutionize industries and redefine societal paradigms.
The hydroxyl group emerges as a beacon of potential, beckoning researchers to push the boundaries of knowledge and creativity.
As they probe its properties and applications, scientists unveil a tapestry of opportunities awaiting exploitation.
From sustainable energy solutions to eco-friendly materials, the hydroxyl group stands poised to catalyze transformative change, propelled by the relentless pursuit of scientific inquiry and technological innovation.
Frequently Asked Questions:
1. What are some examples of compounds containing hydroxyl groups?
Examples include alcohols (such as ethanol and methanol), carbohydrates (like glucose and sucrose), phenols (such as phenol itself), and carboxylic acids (like acetic acid).
2. Compounds containing hydroxyl groups have what properties?
Compounds with hydroxyl groups often exhibit characteristics such as high solubility in water, elevated boiling points due to hydrogen bonding, and unique chemical reactivity influenced by the presence of the -OH group.
3. How do hydroxyl groups contribute to the properties of alcohols?
In alcohols, hydroxyl groups impart distinct physical properties such as odor, volatility, and solubility. Additionally, they influence chemical properties such as acidity, nucleophilicity, and ability to undergo oxidation or reduction reactions.
4. In industrial applications, how are hydroxyl groups utilized?
Hydroxyl groups are utilized in the synthesis of polymers, pharmaceuticals, surfactants, and various other industrial chemicals. Their versatility and reactivity make them valuable building blocks for designing materials with tailored properties and functionalities.
Conclusion:
The hydroxyl group stands as a testament to the remarkable diversity and complexity of organic chemistry. Hydroxyl groups have played pivotal roles in biology, industry, and the environment since their humble beginnings as simple chemical molecules.
Read More: